PT #1 in MIL
Sulfur Hexafluoride
By: Arcinas, Miguel Gio Angelo DM.
Esguerra, Justine Raven L.
Mercado, Janelle Denise B.
Ordonez, Ma. Acel Agatha A.
of XII - Shannon
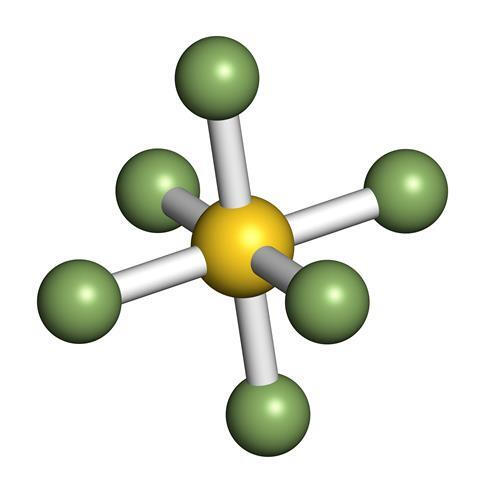
Sulfur Hexafluoride
It is an inorganic compound that is colorless, odorless, non-flammable, and non-toxic gas. It has an octahedral geometry, consisting of six fluorine atoms attached to 1 central sulfur atom.
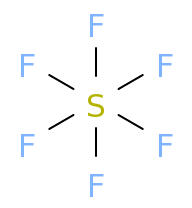
Molecular Formula: SF6
Empirical Formula: SF6
Uses
a test gas in respiratory physiology
an insulating and arc quenching gas in circuit breakers
vitrectomy
electrical transmission and distribution equipment
manufacture of electronics / semiconductors
production of magnesium
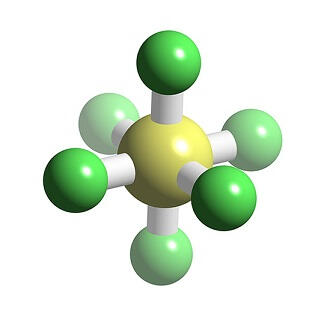
Sulfur Hexafluoride
It is the most potent greenhouse gas currently known, with a global warming potential of 23,900 times that of CO2 over a 100-year period. It has a role as an ultrasound contrast agent and a member of greenhouse gas. Contact with Sulfur Hexafluoride may cause frostbite. Under prolonged exposure to fire or heat, containers may rupture violently and rocket.
Since the 1950s, the U.S. electric power industry has used Sulfur Hexafluoride in circuit breakers, gas-insulated substations, and other switchgear used in the transmission system to manage the high voltages carried between generating stations and customer load centers.The most common use for and largest emission source of Sulfur Hexafluoride, both domestically and internationally, is as an electrical insulator in high voltage equipment that transmits and distributes electricity. The electric power industry uses roughly 80 percent of all SF6 produced worldwide.

Sources:
https://pubchem.ncbi.nlm.nih.gov/compound/Sulfur-hexafluoride#section=Wikidata
https://www.epa.gov/eps-partnership/sulfur-hexafluoride-sf6-basics